WaterDog Outboard Savers
Recruit
- Joined
- Sep 29, 2025
- Messages
- 3
Hello everyone,
My name is Ryan, I am the inventor and founder of the WaterDog Outboard Savers Thermostat Anode that stops internal engine corrosion. I created this after purchasing a boat that had a hole in one of the engines, realizing it must be from the dissimilar metals and then learning about ionic conduction as well as a ton of chemistry.
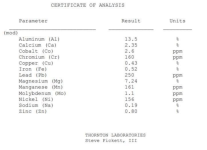
There are a lot of myths surrounding outboard corrosion, the top of which is that the crust that forms inside is made of salt. I took it down to a couple of labs and had it tested and it is mostly aluminum and magnesium hydroxide as well as minerals from the water and there was only 0.19% sodium in the sample, the metals analysis is attached, the other 75% of the sample is minerals and hydroxides.
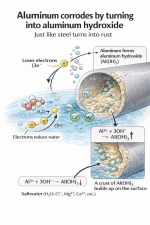
The thermostat and aluminum create what’s called a galvanic or voltaic cell when water is flowing through the system. The thermostat becomes the cathode and the aluminum becomes the anode and the factory anodes are too far with too little surface area in order to protect the aluminum from the thermostat. This is why even if you change all of your anodes every year you still see this build up and your engine still corrodes.
In order to stop this I created an anode that sleeves the thermostat and is submerged in the water, it just slides in the outlet so that both the inside and the outside of the sleeve can corrode in order to protect the aluminum. This means that my sleeve can give up enough material to saturate the surface area of the copper thermostat so that the aluminum doesn’t have to give up any material.
The way that the crust builds up is because electrons leave the aluminum towards the thermostat and interact with the water creating hydroxide. Then the aluminum atom falls into the water because it’s lost its valence electrons and interacts with the hydroxide creating aluminum hydroxide and sticking to the surface creating that white and yellow substance, the yellow is from magnesium in the alloy. Ions move towards the thermostat and get deposited on it as well.
I’m always happy to answer any questions,
Happy boating and long live your engines!
MOD Edit: Read our rules
My name is Ryan, I am the inventor and founder of the WaterDog Outboard Savers Thermostat Anode that stops internal engine corrosion. I created this after purchasing a boat that had a hole in one of the engines, realizing it must be from the dissimilar metals and then learning about ionic conduction as well as a ton of chemistry.
There are a lot of myths surrounding outboard corrosion, the top of which is that the crust that forms inside is made of salt. I took it down to a couple of labs and had it tested and it is mostly aluminum and magnesium hydroxide as well as minerals from the water and there was only 0.19% sodium in the sample, the metals analysis is attached, the other 75% of the sample is minerals and hydroxides.
The thermostat and aluminum create what’s called a galvanic or voltaic cell when water is flowing through the system. The thermostat becomes the cathode and the aluminum becomes the anode and the factory anodes are too far with too little surface area in order to protect the aluminum from the thermostat. This is why even if you change all of your anodes every year you still see this build up and your engine still corrodes.
In order to stop this I created an anode that sleeves the thermostat and is submerged in the water, it just slides in the outlet so that both the inside and the outside of the sleeve can corrode in order to protect the aluminum. This means that my sleeve can give up enough material to saturate the surface area of the copper thermostat so that the aluminum doesn’t have to give up any material.
The way that the crust builds up is because electrons leave the aluminum towards the thermostat and interact with the water creating hydroxide. Then the aluminum atom falls into the water because it’s lost its valence electrons and interacts with the hydroxide creating aluminum hydroxide and sticking to the surface creating that white and yellow substance, the yellow is from magnesium in the alloy. Ions move towards the thermostat and get deposited on it as well.
I’m always happy to answer any questions,
Happy boating and long live your engines!
MOD Edit: Read our rules
Attachments
Last edited by a moderator: