I?ve read all Pascoe?s stuff, some of its pretty good. He seems experienced on many practical aspects, but weak on some things where an engineering background would be useful. Lots of discussion about some of his articles on some other boards that cater to the bigger yacht crowd.
He bases his conclusion on 4 assumptions:
1) There isn't enough air volume within a tank to hold much vapor.
2) On average, tanks are half full, further reducing volume
3) The amount of water vapor in air is very small, even at 100% humidity
4) Conditions aren't right to cause condensation in a fuel tank
I see several problems with his assumptions:
1) There isn't enough air volume within a tank to hold much vapor.
The air volume in the tank doesn?t NEED to hold much vapor. He assumes a closed system (no vent) in his calculations to show that the tank could only contain .81 ounces of water at 86 degrees. While it?s true that the volume of air in the tank at any time can only have a given amount of moisture (the .81 ounces), that volume inside the tank is constantly being exchanged with the outside air through the vent. Any moisture that comes out as dew in the tank is IMMIEDIATLY replaced from the outside atmosphere so the tank atmosphere will ALWAYS have the .81 ounces in it. The tank is vented. The tank has an INFINITE supply of humid air available to it. That humidity is constantly being pulled out by the cool walls of the tank.
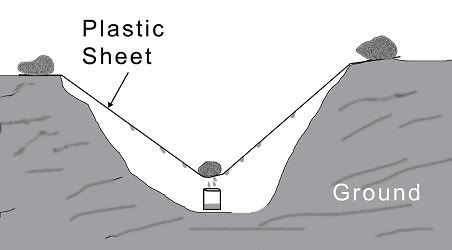
If I wanted to estimate the water that could be trapped, I think I would assume that the tank is the equivalent of the ?Condensation Trap? described in my old Army Survival Manual (It?s just a plastic sheet over a hole in the ground):
The U.S. Army has this to say about condensation traps:
Condensation Trap Efficiency
"It should be noted that condensation traps are not in themselves a sustainable source of water; they are sources for extending or supplementing existing water sources or supplies, and should not be relied on to provide a person's daily requirement for water, since a trap measuring 16" in diameter by 12" deep will only yield around 100 to 150ml per day."
So a trap 16? in diameter by 12? deep will ?only? yield 100 ml a day. A gas tank 16? in diameter and 12? long would hold a little less than 10.5 gallons. Most built in tanks are over 10 gallons in size I bet.
100 ml a day is .03 gallons per day. So 30 days at 100 ml per day would be .9 gallons per month. So even if we assume half this much it could still easily be ? gallon a month of water condensing in the tank.
This fits with my personal experience with 3 cars. I left a 4-Runner with about 1/4 tank of gas in it sitting for a year and the whole bottom of the tank and the electric fuel pump rusted out. It had several inches of water in the bottom. The sides above the fuel were solid rust. Left a Corona sitting with a partial tank for 6 months and the tank rusted up so bad it kept clogging the fuel filter. Shook it around with a bunch of pennies in it to remove the rust then sealed it with Kreem tank sealer. Had a Corvette with half a tank sit there for 6 months and rusted the fuel pump out. Drained a couple of gallons of water out of the tank. Since then I've had several cars sitting around with full tanks and had no other problems, but I also make sure to drive them once a month or so.
2) On average, tanks are half full, further reducing volume.
It?s a valid assumption if you?re looking for average volume. If you?re storing your tank empty, obviously it?s going to have the full surface area and volume of the tank available for condensation.
3) The amount of water vapor in air is very small, even at 100% humidity.
That is true. But there is a lot of air!
4) Conditions aren't right to cause condensation in a fuel tank.
He says:
?In order to condense water out of the atmosphere a surface must be much colder than the air. The problem for the condensation in tank theory is; how do we end up with a fuel tank that is much colder than the air? One way would be to have a very cold day that suddenly warms up dramatically, but when does this ever happen? The weather can turn cold very fast, but does not suddenly get very warm.?
Really weak arguments here. It doesn?t have to be ?much colder?. The moisture comes out of the air at the DEW POINT. Look up ?DEW POINT?:
?The dew point is associated with relative humidity. A high relative humidity indicates that the dew point is closer to the current air temperature. If the relative humidity is 100%, the dew point is equal to the current temperature.?
In other words, at 100% humidity the dew point is equal to the current temperature so the moisture comes out of the air. It?s called ?rain?. One degree of temperature can and will condense moisture out of the air at a high humidity. We see that all the time here in New Orleans. Humidity in the 90?s is not uncommon here. If the humidity is high then a surface doesn?t have to be much cooler than the air to condense the moisture out of it and form dew. If you live in a low humidity area then there is not much moisture in the air and it takes a large temperature difference to get it to condense out. Not sure where Pascoe lives, maybe he?s never seen high humidity.
He really can?t figure out how we get a tank colder than the atmosphere? The tank chills down overnight in the cold air to the air temp (takes a while, several hours probably). Then the atmosphere starts warming up during the day. Not too many built-in tanks exposed to sun to help them warm up. Most tanks are built into the floor and have very little airspace around them. Some even have foam around them. Can you say ?insulation?? Almost the only way an empty tank CAN warm up or cool down is through the atmosphere being exchanged through the vent. So it will take it about the same amount of time to warm up to the atmosphere as it did to cool down. Probably several hours. During that several hours the tank is pumping moisture out of the infinite atmosphere available to it through the vent. I wouldn?t be surprised if on lots of days the tank NEVER gets up to the daytime air temperature and has moisture condensing on its walls all day.
Then he proves engine blocks don?t sweat by saying the underside of the valve covers aren?t rusted? Has he ever noticed that coating of slimy stuff (oil) that?s on the underside of every valve cover I?ve ever seen? My experience with an engine sitting up is that the cylinders above the pistons are the first thing to rust up, probably since the rings scrape most of the oil off them.
Next he says no condensation in the engine. I thought everyone knew you had condensation in an engine. Cold engine block, moist air ? how can you not? Send in an oil sample from any engine and moisture is usually the #1 contaminant! The Fischer Water Titration test is the most common test for water. The explanation below is from MRT Labs (oil analysis).
Karl Fischer Water Titration
"Water is the most common contaminant found in lubricating oils. It is also one of the most damaging to bearings and other lubricated components. It causes corrosion to metal surfaces, lubricant degradation, and poor lubrication. Water can be present in three forms in lubricating oils:
Dissolved: There is a limited amount of solubility of water in oil which is very temperature dependant. At 120? F, about 100 ppm of water can be dissolved in oil. Dissolved water is not harmful nor does it affect the appearance or performance of the lubricant.
Emulsified: Water and oil can form tight bonds that are difficult to break. This form of water in oil is what causes oil to become milky and is the most harmful. Oil will begin to become 'milky' at about 150 - 300 ppm, depending on the base stock and additive in the lubricant.
Free Water: These are free water droplets, often suspended in the lubricant due to surface tension. This form of water in oil is also very harmful to lubricated parts, but is also the easiest to separate."
When you get your engine up to temp it boils most of the moisture off. Some water always remains dissolved in the oil. This is why they recommend short oil change intervals if you use your car for short trips. The reason short trips are considered ?harsh service? is because the oil never gets up to temp to evaporate most of the moisture. NOTE: having ?dissolved moisture? in your oil is not bad! This is NOT the chocolate milk you see from a cracked block or rusted out manifold. On another note, the ?sludge? you see in engines without much maintenance is mainly mildly emulsified oil and water.
If you want to see some people serious about condensation effects look up some articles on aircraft. There?s a REASON a pilot drains his tanks before EVERY flight whether he?s added fuel or not! He doesn?t want to die when the engine quits while trying to burn water! There?s a REASON they make crankcase and cylinder dehumidifiers for aircraft going into storage ? It?s to prevent damage from CONDENSATION in the engine!
In summary, his calculations don?t prove anything. Bad assumptions invalidate them. If I assume that my boat weighs 500 pounds I can calculate that it would go about 100 mph. Doesn?t prove anything. It actually weighs about 4000 pounds, so the calculation is invalid.
I don?t have any experience or knowledge of the ethanol fuels. Sounds like there could be a real concern with them separating. I do know that as long as I?m using real gas I?ll keep my tanks as full as possible!